@article{depienne_click-electrochemistry_2023,
title = {Click-electrochemistry for the rapid labeling of virus, bacteria and cell surfaces},
author = {Sébastien Depienne and Mohammed Bouzelha and Emmanuelle Courtois and Karine Pavageau and Pierre-Alban Lalys and Maia Marchand and Dimitri Alvarez-Dorta and Steven Nedellec and Laura Marín-Fernández and Cyrille Grandjean and Mohammed Boujtita and David Deniaud and Mathieu Mével and Sébastien G. Gouin},
url = {https://doi.org/10.1038/s41467-023-40534-0
https://dx.doi.org/10.26434/chemrxiv-2023-q3sd8
hal-04246348v1 },
doi = {10.1038/s41467-023-40534-0},
issn = {2041-1723},
year = {2023},
date = {2023-08-01},
urldate = {2023-08-01},
journal = {Nature Communications},
volume = {14},
number = {1},
pages = {5122},
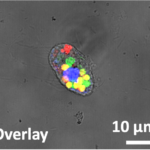
abstract = {Methods for direct covalent ligation of microorganism surfaces remain poorly reported, and mostly based on metabolic engineering for bacteria and cells functionalization. While effective, a faster method avoiding the bio-incorporation step would be highly complementary. Here, we used N-methylluminol (NML), a fully tyrosine-selective protein anchoring group after one-electron oxidation, to label the surface of viruses, living bacteria and cells. The functionalization was performed electrochemically and in situ by applying an electric potential to aqueous buffered solutions of tagged NML containing the viruses, bacteria or cells. The broad applicability of the click-electrochemistry method was explored on recombinant adeno-associated viruses (rAAV2), Escherichia coli (Gram-) and Staphyloccocus epidermidis (Gram + ) bacterial strains, and HEK293 and HeLa eukaryotic cell lines. Surface electro-conjugation was achieved in minutes to yield functionalized rAAV2 that conserved both structural integrity and infectivity properties, and living bacteria and cell lines that were still alive and able to divide.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}