Céline DUC
Maître de conférences Université
section 64
| Équipe : |
Thèmes de recherche
Mécanismes moléculaires du maintien de l’épigénome des micro-algues au cours du cycle cellulaire, Epigénétique/Epigénomique, Bio-informatique
Projets
Parcours universitaire
* 2001-2003 : classe préparatoire BCPST (Biologie Chimie Physique Sciences de la Terre)
* 2003-2006 : Montpellier Sup’Agro
* 2006-2009 : Thèse en Biologie Intégrative des Plantes
* 20020 : HDR – Habilitation à diriger les recherches
Publications
1 publication
Rousselot, Ellyn; Nehr, Zofia; Aury, Jean-Marc; Denoeud, France; Cock, J Mark; Tirichine, Leïla; Duc, Céline
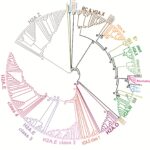
Identification of novel H2A histone variants across diverse clades of algae Article de journal
Dans: Genome Biology, vol. 26, p. 299, 2025.
@article{Rousselot2025,
title = {Identification of novel H2A histone variants across diverse clades of algae},
author = {Ellyn Rousselot and Zofia Nehr and Jean-Marc Aury and France Denoeud and J Mark Cock and Leïla Tirichine and Céline Duc},
editor = {Springer Nature},
year = {2025},
date = {2025-09-23},
urldate = {2025-06-18},
journal = {Genome Biology},
volume = {26},
pages = {299},
abstract = {Background
Histones are among the most conserved proteins in eukaryotes. They not only ensure DNA compaction in the nucleus but also participate in epigenetic regulation of gene expression. These key epigenetic players are divided into replication-coupled histones, expressed during the S-phase, and replication-independent variants, expressed throughout the cell cycle. Compared with other core histones, H2A proteins exhibit a high level of variability but the characterization of algal H2A variants remains very limited.
Results
In this study, we exploit genome and transcriptome data from 22 species to identify H2A variants in brown seaweeds. Combined analyses of phylogenetic data, synteny and protein motifs enable us to reveal the presence of new H2A variants as well as their evolutionary history. We identify three new H2A variants: H2A.N, H2A.O and H2A.E. In brown seaweeds, the H2A.E and H2A.O variants arose from the same monophyletic clade while the H2A.N variant emerged independently. Moreover, the H2A.E variant seems to have a shared ancestry with RC H2A while the H2A.O variant has an H2A.X-characteristic signature without being orthologous to this variant. Based on mass spectrometry, we identify distinct epigenetic marks on these H2A variants. Finally, the H2A.Z, H2A.N and H2A.O from brown seaweeds are ubiquitously expressed while expression of H2A.E has tissue-specific patterns, especially in reproductive tissues.
Conclusions
We thus hypothesize that H2A.O and H2A.X might have convergent functions while H2A.E might fulfil some functions of replication-coupled H2As and/or compensate for the absence of repressive histone marks along with H2A.N.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Histones are among the most conserved proteins in eukaryotes. They not only ensure DNA compaction in the nucleus but also participate in epigenetic regulation of gene expression. These key epigenetic players are divided into replication-coupled histones, expressed during the S-phase, and replication-independent variants, expressed throughout the cell cycle. Compared with other core histones, H2A proteins exhibit a high level of variability but the characterization of algal H2A variants remains very limited.
Results
In this study, we exploit genome and transcriptome data from 22 species to identify H2A variants in brown seaweeds. Combined analyses of phylogenetic data, synteny and protein motifs enable us to reveal the presence of new H2A variants as well as their evolutionary history. We identify three new H2A variants: H2A.N, H2A.O and H2A.E. In brown seaweeds, the H2A.E and H2A.O variants arose from the same monophyletic clade while the H2A.N variant emerged independently. Moreover, the H2A.E variant seems to have a shared ancestry with RC H2A while the H2A.O variant has an H2A.X-characteristic signature without being orthologous to this variant. Based on mass spectrometry, we identify distinct epigenetic marks on these H2A variants. Finally, the H2A.Z, H2A.N and H2A.O from brown seaweeds are ubiquitously expressed while expression of H2A.E has tissue-specific patterns, especially in reproductive tissues.
Conclusions
We thus hypothesize that H2A.O and H2A.X might have convergent functions while H2A.E might fulfil some functions of replication-coupled H2As and/or compensate for the absence of repressive histone marks along with H2A.N.
3 publications
Denoeud, France; Godfroy, Olivier; Cruaud, Corinne; Heesch, Svenja; Nehr, Zofia; Tadrent, Nachida; Couloux, Arnaud; Brillet-Guéguen, Loraine; Delage, Ludovic; Mckeown, Dean; Motomura, Taizo; Sussfeld, Duncan; 0and Lisa Mazéas, Xiao Fan; Terrapon, Nicolas; Barrera-Redondo, Josué; Petroll, Romy; Reynes, Lauric; Choi, Seok-Wan; Jo, Jihoon; Uthanumallian, Kavitha; Bogaert, Kenny; Duc, Céline; Ratchinski, Pélagie; Lipinska, Agnieszka; Noel, Benjamin; Murphy, Eleanor A; 0and Ananya Khatei, Martin Lohr; Hamon-Giraud, Pauline; Vieira, Christophe; Avia, Komlan; 0and Shingo Akita, Svea Sanja Akerfors; Badis, Yacine; Barbeyron, Tristan; Belcour, Arnaud; Berrabah, Wahiba; Blanquart, Samuel; Bouguerba-Collin, Ahlem; Bringloe, Trevor; Cattolico, Rose Ann; Cormier, Alexandre; de Carvalho, Helena Cruz; Dallet, Romain; Clerck, Olivier De; Debit, Ahmed; Denis, Erwan; Destombe, Christophe; Dinatale, Erica; Dittami, Simon; Drula, Elodie; 0and Jeanne Got, Sylvain Faugeron; Graf, Louis; Groisillier, Agnès; Guillemin, Marie-Laure; Harms, Lars; Hatchett, William John; Henrissat, Bernard; Hoarau, Galice; Jollivet, Chloé; Jueterbock, Alexander; Kayal, Ehsan; Knoll, Andrew H; Kogame, Kazuhiro; Bars, Arthur Le; Leblanc, Catherine; Gall, Line Le; 0and Xi Liu, Ronja Ley; LoDuca, Steven T; 0and Philippe Lopez, Pascal Jean Lopez; Manirakiza, Eric; Massau, Karine; Mauger, Stéphane; Mest, Laetitia; Michel, Gurvan; Monteiro, Catia; Nagasato, Chikako; Nègre, Delphine; Pelletier, Eric; Phillips, Naomi; Potin, Philippe; Rensing, Stefan A; Rousselot, Ellyn; Rousvoal, Sylvie; Schroeder, Declan; Scornet, Delphine; Siegel, Anne; Tirichine, Leila; Tonon, Thierry; Valentin, Klaus; Verbruggen, Heroen; Weinberger, Florian; Wheeler, Glen; Kawai, Hiroshi; Peters, Akira F; Yoon, Hwan Su; 0and Naihao Ye, Cécile Hervé; Bapteste, Eric; Valero, Myriam; Markov, Gabriel V; Corre, Erwan; Coelho, Susana M; Wincker, Patrick; Aury, Jean-Marc; Cock, J Mark
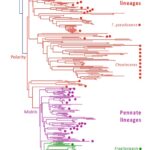
Evolutionary genomics of the emergence of brown algae as key components of coastal ecosystems Article de journal
Dans: Cell, vol. 187, iss. 24, p. 6943-6965, 2024.
@article{nokey,
title = {Evolutionary genomics of the emergence of brown algae as key components of coastal ecosystems},
author = {France Denoeud and Olivier Godfroy and Corinne Cruaud and Svenja Heesch and Zofia Nehr and Nachida Tadrent and Arnaud Couloux and Loraine Brillet-Guéguen and Ludovic Delage and Dean Mckeown and Taizo Motomura and Duncan Sussfeld and Xiao Fan 0and Lisa Mazéas and Nicolas Terrapon and Josué Barrera-Redondo and Romy Petroll and Lauric Reynes and Seok-Wan Choi and Jihoon Jo and Kavitha Uthanumallian and Kenny Bogaert and Céline Duc and Pélagie Ratchinski and Agnieszka Lipinska and Benjamin Noel and Eleanor A Murphy and Martin Lohr 0and Ananya Khatei and Pauline Hamon-Giraud and Christophe Vieira and Komlan Avia and Svea Sanja Akerfors 0and Shingo Akita and Yacine Badis and Tristan Barbeyron and Arnaud Belcour and Wahiba Berrabah and Samuel Blanquart and Ahlem Bouguerba-Collin and Trevor Bringloe and Rose Ann Cattolico and Alexandre Cormier and Helena Cruz de Carvalho and Romain Dallet and Olivier De Clerck and Ahmed Debit and Erwan Denis and Christophe Destombe and Erica Dinatale and Simon Dittami and Elodie Drula and Sylvain Faugeron 0and Jeanne Got and Louis Graf and Agnès Groisillier and Marie-Laure Guillemin and Lars Harms and William John Hatchett and Bernard Henrissat and Galice Hoarau and Chloé Jollivet and Alexander Jueterbock and Ehsan Kayal and Andrew H Knoll and Kazuhiro Kogame and Arthur Le Bars and Catherine Leblanc and Line Le Gall and Ronja Ley 0and Xi Liu and Steven T LoDuca and Pascal Jean Lopez 0and Philippe Lopez and Eric Manirakiza and Karine Massau and Stéphane Mauger and Laetitia Mest and Gurvan Michel and Catia Monteiro and Chikako Nagasato and Delphine Nègre and Eric Pelletier and Naomi Phillips and Philippe Potin and Stefan A Rensing and Ellyn Rousselot and Sylvie Rousvoal and Declan Schroeder and Delphine Scornet and Anne Siegel and Leila Tirichine and Thierry Tonon and Klaus Valentin and Heroen Verbruggen and Florian Weinberger and Glen Wheeler and Hiroshi Kawai and Akira F Peters and Hwan Su Yoon and Cécile Hervé 0and Naihao Ye and Eric Bapteste and Myriam Valero and Gabriel V Markov and Erwan Corre and Susana M Coelho and Patrick Wincker and Jean-Marc Aury and J Mark Cock },
editor = {Cell Press},
url = {https://www.cell.com/cell/fulltext/S0092-8674(24)01272-8?_returnURL=https%3A%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS0092867424012728%3Fshowall%3Dtrue},
doi = {doi: 10.1016/j.cell.2024.10.049},
year = {2024},
date = {2024-11-27},
journal = {Cell},
volume = {187},
issue = {24},
pages = {6943-6965},
abstract = {Brown seaweeds are keystone species of coastal ecosystems, often forming extensive underwater forests, and are under considerable threat from climate change. In this study, analysis of multiple genomes has provided insights across the entire evolutionary history of this lineage, from initial emergence, through later diversification of the brown algal orders, down to microevolutionary events at the genus level. Emergence of the brown algal lineage was associated with a marked gain of new orthologous gene families, enhanced protein domain rearrangement, increased horizontal gene transfer events, and the acquisition of novel signaling molecules and key metabolic pathways, the latter notably related to biosynthesis of the alginate-based extracellular matrix, and halogen and phlorotannin biosynthesis. We show that brown algal genome diversification is tightly linked to phenotypic divergence, including changes in life cycle strategy and zoid flagellar structure. The study also showed that integration of large viral genomes has had a significant impact on brown algal genome content throughout the emergence of the lineage.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Machelová, Adéla; Dadejová, Martina Nešpor; Franek, Michal; Mougeot, Guillaume; Simon, Lauriane; Goff, Samuel Le; Duc, Céline; Bassler, Jasmin; Demko, Martin; Schwarzerová, Jana; Desset, Sophie; Probst, Aline V; Dvořáčková, Martina
The histone chaperones ASF1 and HIRA are required for telomere length and 45S rDNA copy number homeostasis Article de journal
Dans: Plant Journal, vol. 120, iss. 3, p. 1125-1141, 2024.
@article{Machelová2024,
title = {The histone chaperones ASF1 and HIRA are required for telomere length and 45S rDNA copy number homeostasis},
author = {Adéla Machelová and Martina Nešpor Dadejová and Michal Franek and Guillaume Mougeot and Lauriane Simon and Samuel Le Goff and Céline Duc and Jasmin Bassler and Martin Demko and Jana Schwarzerová and Sophie Desset and Aline V Probst and Martina Dvořáčková},
doi = {10.1111/tpj.17041},
year = {2024},
date = {2024-10-14},
journal = {Plant Journal},
volume = {120},
issue = {3},
pages = {1125-1141},
abstract = {Genome stability is significantly influenced by the precise coordination of chromatin complexes that facilitate the loading and eviction of histones from chromatin during replication, transcription, and DNA repair processes. In this study, we investigate the role of the Arabidopsis H3 histone chaperones ANTI-SILENCING FUNCTION 1 (ASF1) and HISTONE REGULATOR A (HIRA) in the maintenance of telomeres and 45S rDNA loci, genomic sites that are particularly susceptible to changes in the chromatin structure. We find that both ASF1 and HIRA are essential for telomere length regulation, as telomeres are significantly shorter in asf1a1b and hira mutants. However, these shorter telomeres remain localized around the nucleolus and exhibit a comparable relative H3 occupancy to the wild type. In addition to regulating telomere length, ASF1 and HIRA contribute to silencing 45S rRNA genes and affect their copy number. Besides, ASF1 supports global heterochromatin maintenance. Our findings also indicate that ASF1 transiently binds to the TELOMERE REPEAT BINDING 1 protein and the N terminus of telomerase in vivo, suggesting a physical link between the ASF1 histone chaperone and the telomere maintenance machinery.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Zarif, Mhammad; Rousselot, Ellyn; Jesus, Bruno; Tirichine, Leïla; Duc, Céline
H3K27me3 and EZH Are Involved in the Control of the Heat-Stress-Elicited Morphological Changes in Diatoms Article de journal
Dans: Int. J. Mol. Sci., vol. 25, iss. 15, p. 8373, 2024.
@article{nokey,
title = {H3K27me3 and EZH Are Involved in the Control of the Heat-Stress-Elicited Morphological Changes in Diatoms},
author = {Mhammad Zarif and Ellyn Rousselot and Bruno Jesus and Leïla Tirichine and Céline Duc},
editor = {MDPI},
url = {https://www.mdpi.com/1422-0067/25/15/8373},
doi = {doi.org/10.3390/ijms25158373},
year = {2024},
date = {2024-07-31},
journal = {Int. J. Mol. Sci.},
volume = {25},
issue = {15},
pages = {8373},
abstract = {Marine water temperatures are increasing due to anthropogenic climate change, constituting a major threat to marine ecosystems. Diatoms are major marine primary producers, and as such, they are subjected to marine heat waves and rising ocean temperatures. Additionally, under low tide, diatoms are regularly exposed to high temperatures. However, physiological and epigenetic responses to long-term exposure to heat stress remain largely unknown in the diatom Phaeodactylum tricornutum. In this study, we investigated changes in cell morphology, photosynthesis, and H3K27me3 abundance (an epigenetic mark consisting of the tri-methylation of lysine 27 on histone H3) after moderate and elevated heat stresses. Mutants impaired in PtEZH—the enzyme depositing H3K27me3—presented reduced growth and moderate changes in their PSII quantum capacities. We observed shape changes for the three morphotypes of P. tricornutum (fusiform, oval, and triradiate) in response to heat stress. These changes were found to be under the control of PtEZH. Additionally, both moderate and elevated heat stresses modulated the expression of genes encoding proteins involved in photosynthesis. Finally, heat stress elicited a reduction of genome-wide H3K27me3 levels in the various morphotypes. Hence, we provided direct evidence of epigenetic control of the H3K27me3 mark in the responses of Phaeodactylum tricornutum to heat stress.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
1 publication
Poulet, Axel; Rousselot, Ellyn; Téletchéa, Stéphane; Noirot, Céline; Jacob, Yannick; Wolfswinkel, Josien; Thiriet, Christophe; Duc, Céline
The Histone Chaperone Network Is Highly Conserved in Physarum polycephalum Article de journal
Dans: International Journal of Molecular Sciences, vol. 24, no. 2, 2023, ISSN: 1422-0067.
@article{ijms24021051,
title = {The Histone Chaperone Network Is Highly Conserved in Physarum polycephalum},
author = {Axel Poulet and Ellyn Rousselot and Stéphane Téletchéa and Céline Noirot and Yannick Jacob and Josien Wolfswinkel and Christophe Thiriet and Céline Duc},
url = {https://www.mdpi.com/1422-0067/24/2/1051
hal-03978828v1 },
doi = {10.3390/ijms24021051},
issn = {1422-0067},
year = {2023},
date = {2023-01-01},
urldate = {2023-01-01},
journal = {International Journal of Molecular Sciences},
volume = {24},
number = {2},
abstract = {The nucleosome is composed of histones and DNA. Prior to their deposition on chromatin, histones are shielded by specialized and diverse proteins known as histone chaperones. They escort histones during their entire cellular life and ensure their proper incorporation in chromatin. Physarum polycephalum is a Mycetozoan, a clade located at the crown of the eukaryotic tree. We previously found that histones, which are highly conserved between plants and animals, are also highly conserved in Physarum. However, histone chaperones differ significantly between animal and plant kingdoms, and this thus probed us to further study the conservation of histone chaperones in Physarum and their evolution relative to animal and plants. Most of the known histone chaperones and their functional domains are conserved as well as key residues required for histone and chaperone interactions. Physarum is divergent from yeast, plants and animals, but PpHIRA, PpCABIN1 and PpSPT6 are similar in structure to plant orthologues. PpFACT is closely related to the yeast complex, and the Physarum genome encodes the animal-specific APFL chaperone. Furthermore, we performed RNA sequencing to monitor chaperone expression during the cell cycle and uncovered two distinct patterns during S-phase. In summary, our study demonstrates the conserved role of histone chaperones in handling histones in an early-branching eukaryote.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
2 publications
Poulet, Axel; Mishra, Laxmi Narayan; Téletchéa, Stéphane; Hayes, Jeffrey J; Jacob, Yannick; Thiriet, Christophe; Duc, Céline
Identification and characterization of histones in Physarum polycephalum evidence a phylogenetic vicinity of Mycetozoans to the animal kingdom Article de journal
Dans: NAR Genomics and Bioinformatics, vol. 3, no. 4, 2021, ISSN: 2631-9268, (lqab107).
@article{10.1093/nargab/lqab107,
title = {Identification and characterization of histones in Physarum polycephalum evidence a phylogenetic vicinity of Mycetozoans to the animal kingdom},
author = {Axel Poulet and Laxmi Narayan Mishra and Stéphane Téletchéa and Jeffrey J Hayes and Yannick Jacob and Christophe Thiriet and Céline Duc},
url = {https://doi.org/10.1093/nargab/lqab107
hal-03595485v1 },
doi = {10.1093/nargab/lqab107},
issn = {2631-9268},
year = {2021},
date = {2021-01-01},
urldate = {2021-01-01},
journal = {NAR Genomics and Bioinformatics},
volume = {3},
number = {4},
abstract = {Physarum polycephalum belongs to Mycetozoans, a phylogenetic clade apart from the animal, plant and fungus kingdoms. Histones are nuclear proteins involved in genome organization and regulation and are among the most evolutionary conserved proteins within eukaryotes. Therefore, this raises the question of their conservation in Physarum and the position of this organism within the eukaryotic phylogenic tree based on histone sequences. We carried out a comprehensive study of histones in Physarum polycephalum using genomic, transcriptomic and molecular data. Our results allowed to identify the different isoforms of the core histones H2A, H2B, H3 and H4 which exhibit strong conservation of amino acid residues previously identified as subject to post-translational modifications. Furthermore, we also identified the linker histone H1, the most divergent histone, and characterized a large number of its PTMs by mass spectrometry. We also performed an in-depth investigation of histone genes and transcript structures. Histone proteins are highly conserved in Physarum and their characterization will contribute to a better understanding of the polyphyletic Mycetozoan group. Our data reinforce that P. polycephalum is evolutionary closer to animals than plants and located at the crown of the eukaryotic tree. Our study provides new insights in the evolutionary history of Physarum and eukaryote lineages.},
note = {lqab107},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Duc, Céline; Thiriet, Christophe
Replication-Coupled Chromatin Remodeling: An Overview of Disassembly and Assembly of Chromatin during Replication Article de journal
Dans: International Journal of Molecular Sciences, vol. 22, no. 3, 2021, ISSN: 1422-0067.
@article{ijms22031113,
title = {Replication-Coupled Chromatin Remodeling: An Overview of Disassembly and Assembly of Chromatin during Replication},
author = {Céline Duc and Christophe Thiriet},
url = {https://www.mdpi.com/1422-0067/22/3/1113
hal-04210949v1 },
doi = {10.3390/ijms22031113},
issn = {1422-0067},
year = {2021},
date = {2021-01-01},
urldate = {2021-01-01},
journal = {International Journal of Molecular Sciences},
volume = {22},
number = {3},
abstract = {The doubling of genomic DNA during the S-phase of the cell cycle involves the global remodeling of chromatin at replication forks. The present review focuses on the eviction of nucleosomes in front of the replication forks to facilitate the passage of replication machinery and the mechanism of replication-coupled chromatin assembly behind the replication forks. The recycling of parental histones as well as the nuclear import and the assembly of newly synthesized histones are also discussed with regard to the epigenetic inheritance.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
1 publication
Duc, Céline; Yoth, Marianne; Jensen, Silke; Mouniée, Nolwenn; Bergman, Casey M; Vaury, Chantal; Brasset, Emilie
Trapping a somatic endogenous retrovirus into a germline piRNA cluster immunizes the germline against further invasion Article de journal
Dans: Genome Biology, vol. 20, no. 1, p. 127, 2019, ISSN: 1474-760X.
@article{duc_trapping_2019,
title = {Trapping a somatic endogenous retrovirus into a germline piRNA cluster immunizes the germline against further invasion},
author = {Céline Duc and Marianne Yoth and Silke Jensen and Nolwenn Mouniée and Casey M Bergman and Chantal Vaury and Emilie Brasset},
url = {https://genomebiology.biomedcentral.com/articles/10.1186/s13059-019-1736-x},
doi = {10.1186/s13059-019-1736-x},
issn = {1474-760X},
year = {2019},
date = {2019-01-01},
urldate = {2019-01-01},
journal = {Genome Biology},
volume = {20},
number = {1},
pages = {127},
abstract = {Background: For species survival, the germline must faithfully transmit genetic information to the progeny. Transposable elements (TEs) constitute a significant threat to genome stability due to their mobility. In the metazoan germline, their mobilization is limited by a class of small RNAs called PIWI-interacting RNAs (piRNAs) produced by dedicated genomic loci called piRNA clusters. Although the piRNA pathway is an adaptive genomic immunity system, it remains unclear how the germline gains protection from a new transposon invasion.
Results: To address this question, we analyze Drosophila melanogaster lines harboring a deletion within flamenco, a major piRNA cluster specifically expressed in somatic follicular cells. This deletion leads to derepression of the retrotransposon ZAM in the somatic follicular cells and subsequent germline genome invasion. In this mutant line, we identify de novo production of sense and antisense ZAM-derived piRNAs that display a germinal molecular signature. These piRNAs originated from a new ZAM insertion into a germline dual-strand piRNA cluster and silence ZAM expression specifically in germ cells. Finally, we find that ZAM trapping in a germinal piRNA cluster is a frequent event that occurs early during the isolation of the mutant line.
Conclusions: Transposons can hijack the host developmental process to propagate whenever their silencing is lost. Here, we show that the germline can protect itself by trapping invading somatic-specific TEs into germline piRNA clusters. This is the first demonstration of “auto-immunization” of a germline endangered by mobilization of a surrounding somatic TE.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Results: To address this question, we analyze Drosophila melanogaster lines harboring a deletion within flamenco, a major piRNA cluster specifically expressed in somatic follicular cells. This deletion leads to derepression of the retrotransposon ZAM in the somatic follicular cells and subsequent germline genome invasion. In this mutant line, we identify de novo production of sense and antisense ZAM-derived piRNAs that display a germinal molecular signature. These piRNAs originated from a new ZAM insertion into a germline dual-strand piRNA cluster and silence ZAM expression specifically in germ cells. Finally, we find that ZAM trapping in a germinal piRNA cluster is a frequent event that occurs early during the isolation of the mutant line.
Conclusions: Transposons can hijack the host developmental process to propagate whenever their silencing is lost. Here, we show that the germline can protect itself by trapping invading somatic-specific TEs into germline piRNA clusters. This is the first demonstration of “auto-immunization” of a germline endangered by mobilization of a surrounding somatic TE.
2019
Benoit, Matthias; Simon, Lauriane; Desset, Sophie; Duc, Céline; Cotterell, Sylviane; Poulet, Axel; Goff, Samuel Le; Tatout, Christophe; Probst, Aline V
Replication-coupled histone H3.1 deposition determines nucleosome composition and heterochromatin dynamics during Arabidopsis seedling development Article de journal
Dans: New Phytologist, vol. 221, no. 1, p. 385-398, 2019.
@article{EQ4:DUC:2019b,
title = {Replication-coupled histone H3.1 deposition determines nucleosome composition and heterochromatin dynamics during Arabidopsis seedling development},
author = {Matthias Benoit and Lauriane Simon and Sophie Desset and Céline Duc and Sylviane Cotterell and Axel Poulet and Samuel Le Goff and Christophe Tatout and Aline V Probst},
url = {https://nph.onlinelibrary.wiley.com/doi/abs/10.1111/nph.15248},
doi = {10.1111/nph.15248},
year = {2019},
date = {2019-01-01},
journal = {New Phytologist},
volume = {221},
number = {1},
pages = {385-398},
abstract = {Summary Developmental phase transitions are often characterized by changes in the chromatin landscape and heterochromatin reorganization. In Arabidopsis, clustering of repetitive heterochromatic loci into so-called chromocenters is an important determinant of chromosome organization in nuclear space. Here, we investigated the molecular mechanisms involved in chromocenter formation during the switch from a heterotrophic to a photosynthetically competent state during early seedling development. We characterized the spatial organization and chromatin features at centromeric and pericentromeric repeats and identified mutant contexts with impaired chromocenter formation. We find that clustering of repetitive DNA loci into chromocenters takes place in a precise temporal window and results in reinforced transcriptional repression. Although repetitive sequences are enriched in H3K9me2 and linker histone H1 before repeat clustering, chromocenter formation involves increasing enrichment in H3.1 as well as H2A.W histone variants, hallmarks of heterochromatin. These processes are severely affected in mutants impaired in replication-coupled histone assembly mediated by CHROMATIN ASSEMBLY FACTOR 1 (CAF-1). We further reveal that histone deposition by CAF-1 is required for efficient H3K9me2 enrichment at repetitive sequences during chromocenter formation. Taken together, we show that chromocenter assembly during post-germination development requires dynamic changes in nucleosome composition and histone post-translational modifications orchestrated by the replication-coupled H3.1 deposition machinery.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Froussios, Kimon; Schurch, Nick J; Mackinnon, Katarzyna; Gierliṅski, Marek; Duc, Céline; Simpson, Gordon G; Barton, Geoffrey J
How well do RNA-Seq differential gene expression tools perform in a complex eukaryote? A case study in Arabidopsis thaliana Article de journal
Dans: Bioinformatics, vol. 35, no. 18, p. 3372-3377, 2019, ISSN: 1367-4803.
@article{EQ4:DUC:2019c,
title = {How well do RNA-Seq differential gene expression tools perform in a complex eukaryote? A case study in Arabidopsis thaliana},
author = {Kimon Froussios and Nick J Schurch and Katarzyna Mackinnon and Marek Gierliṅski and Céline Duc and Gordon G Simpson and Geoffrey J Barton},
url = {https://doi.org/10.1093/bioinformatics/btz089},
doi = {10.1093/bioinformatics/btz089},
issn = {1367-4803},
year = {2019},
date = {2019-01-01},
journal = {Bioinformatics},
volume = {35},
number = {18},
pages = {3372-3377},
abstract = {RNA-seq experiments are usually carried out in three or fewer replicates. In order to work well with so few samples, differential gene expression (DGE) tools typically assume the form of the underlying gene expression distribution. In this paper, the statistical properties of gene expression from RNA-seq are investigated in the complex eukaryote, Arabidopsis thaliana, extending and generalizing the results of previous work in the simple eukaryote Saccharomyces cerevisiae.We show that, consistent with the results in S.cerevisiae, more gene expression measurements in A.thaliana are consistent with being drawn from an underlying negative binomial distribution than either a log-normal distribution or a normal distribution, and that the size and complexity of the A.thaliana transcriptome does not influence the false positive rate performance of nine widely used DGE tools tested here. We therefore recommend the use of DGE tools that are based on the negative binomial distribution.The raw data for the 17 WT Arabidopsis thaliana datasets is available from the European Nucleotide Archive (E-MTAB-5446). The processed and aligned data can be visualized in context using IGB (Freese et al., 2016), or downloaded directly, using our publicly available IGB quickload server at https://compbio.lifesci.dundee.ac.uk/arabidopsisQuickload/public_quickload/ under ^a€˜RNAseq>Froussios2019^a€texttrademark. All scripts and commands are available from github at https://github.com/bartongroup/KF_arabidopsis-GRNA.Supplementary data are available at Bioinformatics online.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
2017
Poulet, Axel; Duc, Céline; Voisin, Maxime; Desset, Sophie; Tutois, Sylvie; Vanrobays, Emmanuel; Benoit, Matthias; Evans, David E; Probst, Aline V; Tatout, Christophe
The LINC complex contributes to heterochromatin organisation and transcriptional gene silencing in plants Article de journal
Dans: Journal of Cell Science, vol. 130, no. 3, p. 590–601, 2017, ISSN: 0021-9533.
@article{EQ4:DUC:2017,
title = {The LINC complex contributes to heterochromatin organisation and transcriptional gene silencing in plants},
author = {Axel Poulet and Céline Duc and Maxime Voisin and Sophie Desset and Sylvie Tutois and Emmanuel Vanrobays and Matthias Benoit and David E Evans and Aline V Probst and Christophe Tatout},
url = {https://jcs.biologists.org/content/130/3/590},
doi = {10.1242/jcs.194712},
issn = {0021-9533},
year = {2017},
date = {2017-01-01},
journal = {Journal of Cell Science},
volume = {130},
number = {3},
pages = {590--601},
publisher = {The Company of Biologists Ltd},
abstract = {The linker of nucleoskeleton and cytoskeleton (LINC) complex is an evolutionarily well-conserved protein bridge connecting the cytoplasmic and nuclear compartments across the nuclear membrane. While recent data support its function in nuclear morphology and meiosis, its involvement in chromatin organisation has not been studied in plants. Here, 3D imaging methods have been used to investigate nuclear morphology and chromatin organisation in interphase nuclei of the model plant Arabidopsis thaliana in which heterochromatin clusters in conspicuous chromatin domains called chromocentres. Chromocentres form a repressive chromatin environment contributing to transcriptional silencing of repeated sequences, a general mechanism needed for genome stability. Quantitative measurements of the 3D position of chromocentres indicate their close proximity to the nuclear periphery but that their position varies with nuclear volume and can be altered in specific mutants affecting the LINC complex. Finally, we propose that the plant LINC complex contributes to proper heterochromatin organisation and positioning at the nuclear periphery, since its alteration is associated with the release of transcriptional silencing as well as decompaction of heterochromatic sequences.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Duc, Céline; Benoit, Matthias; Détourné, Gwénaëlle; Simon, Lauriane; Poulet, Axel; Jung, Matthieu; Veluchamy, Alaguraj; Latrasse, David; Goff, Samuel Le; Cotterell, Sylviane; Tatout, Christophe; Benhamed, Moussa; Probst, Aline V
Arabidopsis ATRX Modulates H3.3 Occupancy and Fine-Tunes Gene Expression Article de journal
Dans: The Plant Cell, vol. 29, no. 7, p. 1773–1793, 2017, ISSN: 1040-4651.
@article{EQ4:DUC:2017b,
title = {Arabidopsis ATRX Modulates H3.3 Occupancy and Fine-Tunes Gene Expression},
author = {Céline Duc and Matthias Benoit and Gwénaëlle Détourné and Lauriane Simon and Axel Poulet and Matthieu Jung and Alaguraj Veluchamy and David Latrasse and Samuel Le Goff and Sylviane Cotterell and Christophe Tatout and Moussa Benhamed and Aline V Probst},
url = {http://www.plantcell.org/content/29/7/1773},
doi = {10.1105/tpc.16.00877},
issn = {1040-4651},
year = {2017},
date = {2017-01-01},
journal = {The Plant Cell},
volume = {29},
number = {7},
pages = {1773--1793},
publisher = {American Society of Plant Biologists},
abstract = {Histones are essential components of the nucleosome, the major chromatin subunit that structures linear DNA molecules and regulates access of other proteins to DNA. Specific histone chaperone complexes control the correct deposition of canonical histones and their variants to modulate nucleosome structure and stability. In this study, we characterize the Arabidopsis thaliana Alpha Thalassemia-mental Retardation X-linked (ATRX) ortholog and show that ATRX is involved in histone H3 deposition. Arabidopsis ATRX mutant alleles are viable, but show developmental defects and reduced fertility. Their combination with mutants of the histone H3.3 chaperone HIRA (Histone Regulator A) results in impaired plant survival, suggesting that HIRA and ATRX function in complementary histone deposition pathways. Indeed, ATRX loss of function alters cellular histone H3.3 pools and in consequence modulates the H3.1/H3.3 balance in the cell. H3.3 levels are affected especially at genes characterized by elevated H3.3 occupancy, including the 45S ribosomal DNA (45S rDNA) loci, where loss of ATRX results in altered expression of specific 45S rDNA sequence variants. At the genome-wide scale, our data indicate that ATRX modifies gene expression concomitantly to H3.3 deposition at a set of genes characterized both by elevated H3.3 occupancy and high expression. Together, our results show that ATRX is involved in H3.3 deposition and emphasize the role of histone chaperones in adjusting genome expression.GlossaryHUhydroxyureaChIPchromatin immunoprecipitationTEstransposable elementsGOGene OntologyRPKMreads per kilobase per million mapped readsNORnucleolus organizer region},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
2015
Duc, Céline; Benoit, Matthias; Goff, Samuel Le; Simon, Lauriane; Poulet, Axel; Cotterell, Sylviane; Tatout, Christophe; Probst, Aline V
The histone chaperone complex HIR maintains nucleosome occupancy and counterbalances impaired histone deposition in CAF-1 complex mutants Article de journal
Dans: The Plant Journal, vol. 81, no. 5, p. 707-722, 2015.
@article{EQ4:DUC:2015,
title = {The histone chaperone complex HIR maintains nucleosome occupancy and counterbalances impaired histone deposition in CAF-1 complex mutants},
author = {Céline Duc and Matthias Benoit and Samuel Le Goff and Lauriane Simon and Axel Poulet and Sylviane Cotterell and Christophe Tatout and Aline V Probst},
url = {https://onlinelibrary.wiley.com/doi/abs/10.1111/tpj.12758},
doi = {10.1111/tpj.12758},
year = {2015},
date = {2015-01-01},
journal = {The Plant Journal},
volume = {81},
number = {5},
pages = {707-722},
abstract = {Summary Chromatin organization is essential for coordinated gene expression, genome stability, and inheritance of epigenetic information. The main components involved in chromatin assembly are specific complexes such as Chromatin Assembly Factor 1 (CAF-1) and Histone Regulator (HIR), which deposit histones in a DNA synthesis-dependent or -independent manner, respectively. Here, we characterize the role of the plant orthologs Histone Regulator A (HIRA), Ubinuclein (UBN) and Calcineurin Binding protein 1 (CABIN1), which constitute the HIR complex. Arabidopsis loss-of-function mutants for the various subunits of the complex are viable, but hira mutants show reduced fertility. We show that loss of HIRA reduces extractable histone H3 protein levels and decreases nucleosome occupancy at both actively transcribed genes and heterochromatic regions. Concomitantly, HIRA contributes to maintenance of silencing of pericentromeric repeats and certain transposons. A genetic analysis based on crosses between mutants deficient in subunits of the CAF-1 and HIR complexes showed that simultaneous loss of both the CAF-1 and HIR histone H3 chaperone complexes severely affects plant survival, growth and reproductive development. Our results suggest that HIRA partially rescues impaired histone deposition in fas mutants to preserve nucleosome occupancy, implying plasticity in histone variant interaction and deposition.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Stagiaires encadrés :
- EMIE LEFEVRE, Master 2 Sciences de la Mer, Interactions biotiques et Perturbations Anthropiques en environnement marin, Université de Toulon
- Valentin PLANTIER, master GGBS, Nantes Université
- Ellyn ROUSSELOT, master GGBS, Nantes Université